In biotech this year, you will need to prepare solutions so this lab activity is a means to help you understand how to make various types of solutions.
1. Using concentrated copper (II) sulfate as your stock solution, how did you prepare 5 mL of diluted copper (II) sulfate using a 1:5 dilution factor?


2. Imagine you purchased some 50x Tris buffer. How would you make 250 mL of diluted Tris buffer?

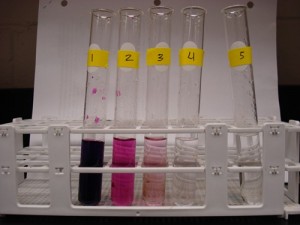
3. This picture represents the dilution series you prepared with potassium permanganate. How many mililiters of solution are in test tube #2 after you complete the dilution series? What about test tube #5? What fraction of stock solution is represented in test tube 2?
4. Let’s say you need to make 250 mL of a 0.4 M solution of sodium chloride. How would you do this?
5. An experiment calls for 50 mL of a 14% sodium chloride solution. How will you prepare this solution?

